3MAUTO: NVD-003
3MAUTO: NVD-003
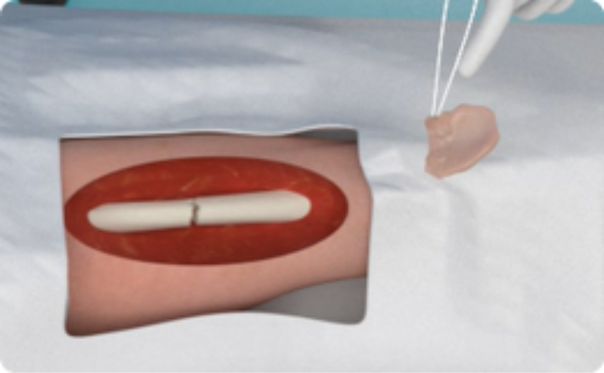
NVD-003 is a single treatment for critical size bone reconstruction, which is comprised of differentiated stem cells within an extracellular matrix resulting in active local delivery of highly specific growth factors and miRNAs leading to biological activity necessary for tissue regeneration for large bone defects. The active cells in this autologous graft help achieve union in critical size bone defects.
The lead indication for NVD-003 is congenital pseudarthrosis of the tibia (CPT), a rare pediatric orthopedic condition in which the tibia of a young child breaks due to bowing and does not heal, resulting in non-union. CPT is an orphan indication in the US and Novadip was granted a Priority Review Voucher from the US FDA, which will be granted upon the potential FDA approval of NVD-003 in CPT.
NVD-003 – A Phase 1b/2a trial in adults with bone non-union is closed with results shared in 2022. A Phase 1b/2a clinical trial in CPT began in the US and EU in 2022.
Congenital pseudarthrosis of the tibia (CPT) is a rare pediatric bone development disorder most often identified before a child turns two years old. This impacts patients in just .5-3.5 in 150,0001, 2, 3 live births and the condition is very rare and debilitating. CPT occurs when a shin bone fracture does not properly heal, resulting in non-union and forms a false joint (pseudoarthrosis). This can happen spontaneously or from a minor traumatic injury.
The current standard of care is surgery, with autologous bone graft, which is associated with poor long-term outcomes, high risk of chronic pain, infection, blood loss/transfusion, and persistent risk of amputation.
References:
1. Khan T, Bone Joint J. 2013, PMID: 23908415
2. Heikkinen ES, Acta Orthop Scand. 1999, PMID: 10429605
3. Hefti F, J Pediatr Orthop B. 2000, PMID: 10647103
Autologous ASCs for critical bone and large tissue reconstruction
Adipose collection (autologous)

3D Product Manufacturing
NVD-003
Moldable, cellular, large volume (>10cc) graft for direct implantation- Cell collection to implantation in 3 months
- Robust osteogenic capacity driven by adipose stem cells
- Balanced “cocktail” of growth factors needed to drive critical size tissue repair
- Enriched in specific bioactive molecules to drive bone-healing pathways
NVD-003: A Potential Single Treatment Cure of Rare Pediatric Bone Defects
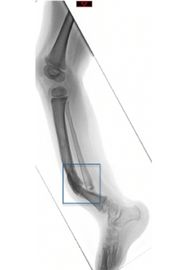
CPT leading to high risk of amputation
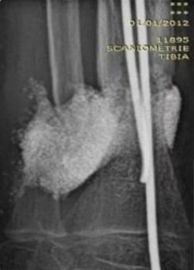
Autologous implant mimics & accelerates natural healing

- Potential single treatment to save limb/restore mobility
- Active cells in autologous graft help achieve union in critical size defects
- Achieved durable bone fusion at 24 months
- 13 patients fully mobile at up to 4 year follow up
- Compassionate use in 4 CPT patients supported IND submission to the US FDA
Orphan Drug and Rare Pediatric Disease Designations
The NVD-003 autologous program was granted Orphan Drug and Rare Pediatric Disease designation by the US Food and Drug Administration (FDA) in 2020 for CPT. Following both designations and a successful potential FDA approval in CPT, Novadip will be eligible to obtain a priority review voucher when the product reaches the market. The priority review voucher is an asset valued at more than $100 million.
Our Science
Novadip is developing a new class of regenerative tissue products that accelerate healing in a single treatment.
Patients
Learn how we are transforming the lives of patients.
What’s New
Stay updated on the latest news from Novadip, including corporate and clinical announcements.